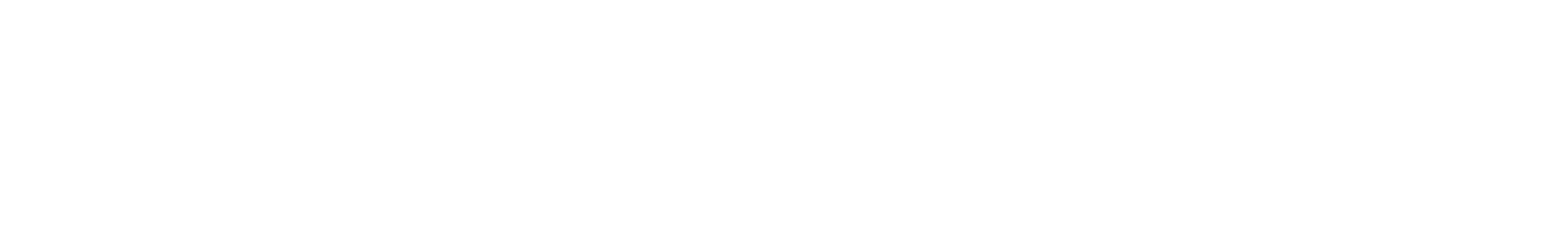U.S. Health and Human Services Secretary Robert F. Kennedy Jr., testifies before a Senate Finance Committee hearing on President Donald Trump’s 2026 health care agenda, on Capitol Hill in Washington, D.C., U.S., September 4, 2025.
Jonathan Ernst | Reuters
President Donald Trump on Tuesday signed a memorandum that aims to crack down on direct-to-consumer prescription drug ads, in a move that stops short of an outright ban.
The pharmaceutical industry’s ability to advertise drugs directly to consumers has long been a target of Health and Human Services Secretary Robert F. Kennedy Jr., who floated a ban on those ads in November and has argued that they lead Americans to use more prescription medications. But senior administration officials on Tuesday said the new memorandum goes beyond drugmakers and traditional TV advertising by also targeting ads on social media and digital platforms.
The measure directs Kennedy and Food and Drug Administration Commissioner Marty Makary to take actions to ensure transparency and accuracy in drug advertising, including by increasing the amount of information related to any product risks, officials said.
“Our goal is to ensure that patients have proper information about drugs that have potential harms, and it’s to rebuild public trust,” one official said, adding that the administration plans to use existing regulations on ads rather than seek new rules.
The Trump administration will send out around 100 cease-and-desist enforcement letters and thousands of warning letters alerting companies that the government plans to enforce current regulations around those ads, the officials told reporters. The administration also plans to scrutinize social media companies as well as influencers paid to promote pharmaceutical products without proper disclosures or without following the rules that drugmakers must follow, according to the officials.
“There has been broad frustration with the increasing prevalence of these ads creating a misleading impression, specifically not disclosing side effects appropriately – ads that have encroached now into social media without proper disclosures, and ads of online pharmacies that are not following the same rules that many pharmaceutical companies follow,” one official told reporters.
The Trump administration will not publish the letters it is sending out, but “we can certainly look at doing that,” one official said. The official said no company stood out as a major perpetrator.
But they noted that senators earlier this year wrote a letter to the FDA about a Hims & Hers Super Bowl ad in February that promoted weight loss drugs from its online pharmacy “with really no mention of any potential harms.”
“That certainly caught a lot of people’s eyes,” the official said.
The administration also plans to eliminate what officials called a “loophole” from a 1997 FDA action, which allowed drug ads to refer consumers to another source, such as a website, for full information on a pharmaceutical product’s side effects or potential risks. Before that action, the FDA established strict guidelines for TV drug advertising in 1985, requiring that they include all of the drug’s possible side effects if they want to indicate the condition the product is intended to treat.
One official said enforcement has faltered in recent years, so the Trump administration is “going to take these regulations seriously and respond to the call from physicians.” Officials said many U.S. physicians don’t believe drug ads contain balanced information, and argue that they can distort their relationships with patients.
One official added that a few large pharmaceutical company CEOs have told the administration to take action on drug advertising, without disclosing the names of specific firms.
The memorandum comes after the release of a report from Trump’s “Make America Healthy Again” commission on Tuesday, which says HHS, the FDA, the Federal Trade Commission and the Department of Justice will ramp up oversight and enforcement of prescription drug advertising laws. Violations “demonstrating harm” will be prioritized, including by social media influencers and direct-to-consumer telehealth companies.
Trump attempted to rein in pharmaceutical industry advertising during his first term, issuing a regulation in 2019 that would have required drugmakers to include their list prices in TV ads. A federal judge nixed that effort, saying HHS had overstepped its authority.
Drug advertising has taken off since the FDA relaxed rules around drug advertising in 1997. Pharma advertisers spent more than $10 billion on prescription drug ads last year, according to several reports citing MediaRadar data. The top 10 medications were responsible for about a third of that spending in 2024.